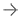July 11, 2016 — The U.S. Food and Drug Administration (FDA) recently approved the firstfocused ultrasounddevice to treat essential tremor in patients who have not responded to medication. ExAblate Neuro usesmagnetic resonance (MR) imagestaken during the procedure to deliver focused ultrasound to destroy brain tissue in a tiny area thought to be responsible for causing tremors.
“Patients with essential tremor who have not seen improvement with medication now have a new treatment option that could help them to avoid more invasive surgical treatments,” said Carlos Peña, Ph.D., M.S., director of the division of neurological and physical medicine devices in the FDA’s Center for Devices and Radiological Health. “As with other treatments for essential tremor, this new device is not a cure but could help patients enjoy a better quality of life.”
特发性震颤,又称良性特发性震颤,是最常见的震颤形式。根据美国国家神经疾病和中风研究所的数据,数百万美国人,通常是40岁以上的人,受到这种疾病的影响。特发性震颤可以用-受体阻滞剂或抗惊厥药物治疗。如果药物治疗不能控制症状,也可以通过手术(丘脑切开术)或深部脑刺激装置来破坏大脑中控制一些不自主运动的微小部分(丘脑)。
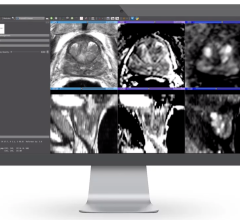
To determine if the ExAblate Neuro treatment is appropriate, patients should first have MR and computerized tomography (CT) scans. Those undergoing treatment with the MRI-guided device lie in an MRI scanner that takes images to help a doctor identify the targeted area in the brain’s thalamus for treatment. Treatment with transcranial focused ultrasound energy is administered with incremental increases in energy until patients achieve a reduction of tremor. Patients are awake and responsive during the entire treatment.
Data supporting the safety and effectiveness of the device system included a double-blind control trial involving 76 patients with essential tremor who had not responded to medication therapy. Fifty-six of the patients were randomly selected to receive the ExAblate Neuro treatment and 20 received a fake treatment. Patients in the control group were able to cross over into the treatment group three months later.
接受ExAblate neuroo治疗的患者在治疗三个月后的震颤和运动功能(复合震颤/运动功能评分)与基线评分相比改善了近50%。对照组患者没有改善,一些患者在假手术后出现轻微恶化,然后转入治疗组。术后12个月,治疗组的这些分数与基线相比仍有40%的改善。
Adverse events for the ExAblate Neuro are consistent with those reported for thalamotomy surgery, including numbness/tingling of the fingers, headache, imbalance/unsteadiness, loss of control of body movements (ataxia) or gait disturbance. Other side effects identified as possibly related to treatment with MR-guided focused ultrasound treatments include tissue damage in an area other than the treatment area, hemorrhage in the treated area requiring emergency treatment, skin burns with ulceration of the skin, skin retraction and scar formation and blood clots.
The ExAblate Neuro treatment is contraindicated for patients who cannot have MRI, including those who have a non-MRI compatible implanted metallic device such as a cardiac pacemaker, those with allergies to MR contrast agents or those with body size limitations for MR.
The treatment should also not be used in women who are pregnant, patients with advanced kidney disease or on dialysis, those with unstable heart conditions or severe hypertension, patients exhibiting any behavior consistent with ethanol or substance abuse or patients with a history of abnormal bleeding, hemorrhage and/or blood clotting disorders (coagulopathy). Patients currently taking anticoagulant drugs or drugs known to increase the risk of hemorrhage, patients with a history of cerebrovascular disease (strokes) or brain tumors and patients who are not able to tolerate the prolonged stationary position during treatment also should not have the procedure.
ExAblate Neuro is manufactured by InSightec in Dallas, Texas.
For more information:www.insightec.com


 August 10, 2022
August 10, 2022