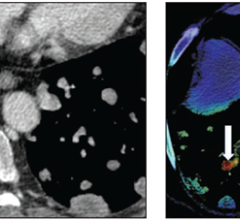
September 22, 2021 — The U.S. Food and Drug Administration authorized marketing of software to assist medical professionals who examine body tissues (pathologists) in the detection of areas that are suspicious for cancer as an adjunct (supplement) to the review of digitally-scanned slide images from prostate biopsies (tissue removed from the body). The software, calledPaige Prostate, is the firstartificial intelligence(AI)-based software designed to identify an area of interest on the prostate biopsy image with the highest likelihood of harboring cancer so it can be reviewed further by the pathologist if the area of concern has not been identified on initial review.
“Pathologists examine biopsies of tissue suspected for diseases, such as prostate cancer, every day. Identifying areas of concern on the biopsy image can help pathologists make a diagnosis that informs the appropriate treatment,” saidTim Stenzel, M.D., Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health. “The authorization of this AI-based software can help increase the number of identified prostate biopsy samples with cancerous tissue, which can ultimately save lives.”
Cancer that starts in the prostate is calledprostate cancer. According to theCenters for Disease Control and Prevention, aside from non-melanoma skin cancer, prostate cancer is the most common cancer among men in the United States. It is also one of the leading causes of cancer death among men.
Paige Prostate is compatible for use with slide images that have been digitized using a scanner. The digitized slide image can then be visualized using a slide image viewer.
FDA评估了一项临床研究的数据,在这项研究中,16名病理学家使用扫描仪对527张前列腺活检切片(171张癌切片和356张良性切片)进行了数字化检查。对于每张幻灯片图像,每位病理学家完成两次评估,一次没有Paige前列腺的帮助(无辅助阅读),一次有Paige前列腺的帮助(辅助阅读)。虽然临床研究没有评估对患者最终诊断的影响,这通常是基于多次活检,但研究发现,与病理学家对单个活检的整张幻灯片图像的无辅助读取相比,Paige前列腺对单个幻灯片图像的癌症检测平均提高了7.3%,而对良性幻灯片图像的读取没有影响。
Potential risks include false negative and false positive results, which is mitigated by the device’s use as an adjunct (e.g., the device assists pathologists reviewing slide images) and by the professional evaluation by a qualified pathologist who takes into account patient history among other relevant clinical information, and who may perform additional laboratory studies on the samples prior to rendering a final diagnosis.
The FDA reviewed the device through theDe Novopremarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. Along with this authorization, the FDA is establishing special controls for devices of this type, including requirements related to labeling and performance testing. When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type. This action creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device.
The FDA granted marketing authorization of the Paige Prostate software to Paige.AI.
For more information:www.fda.gov


 August 01, 2022
August 01, 2022